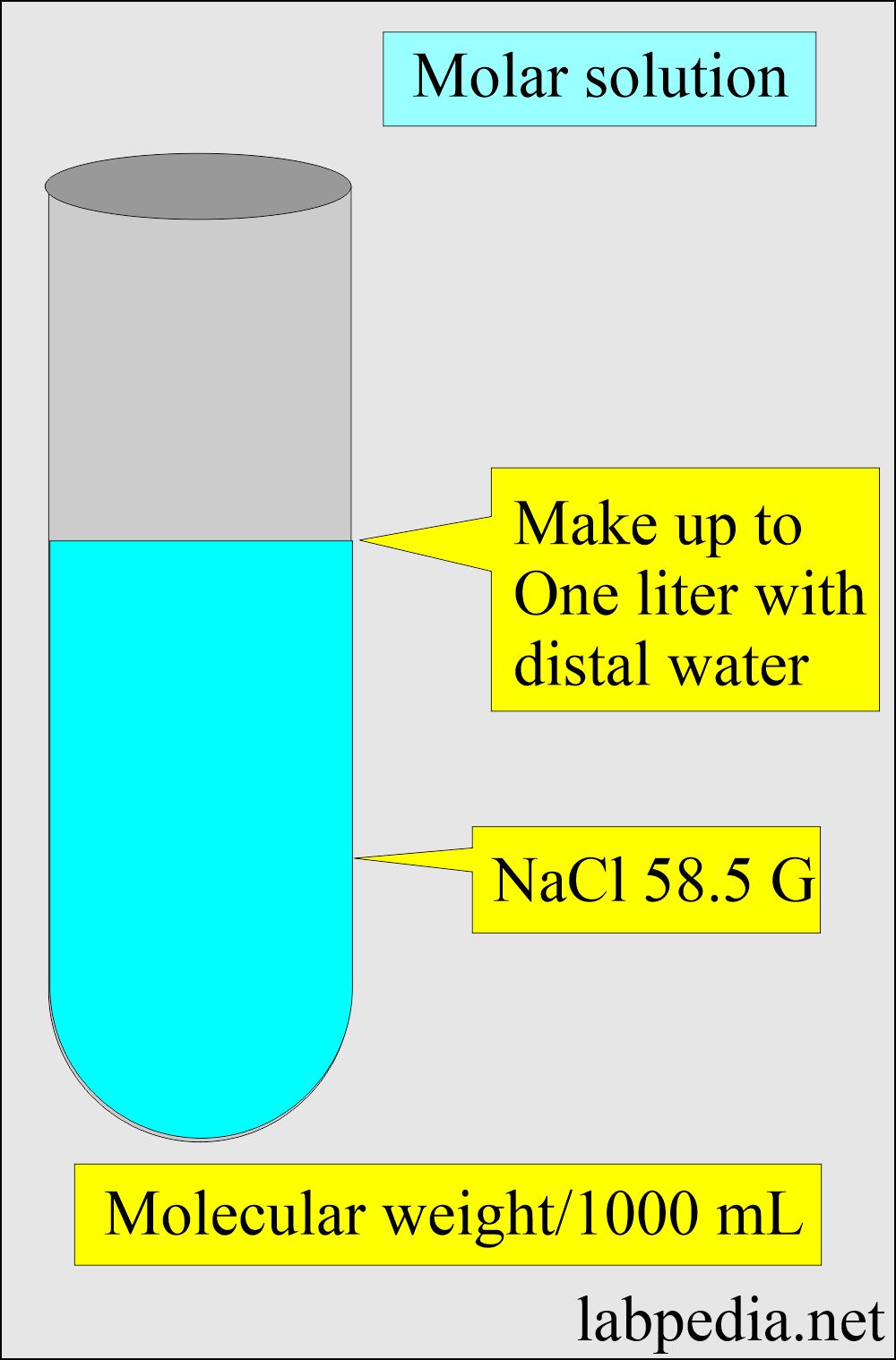
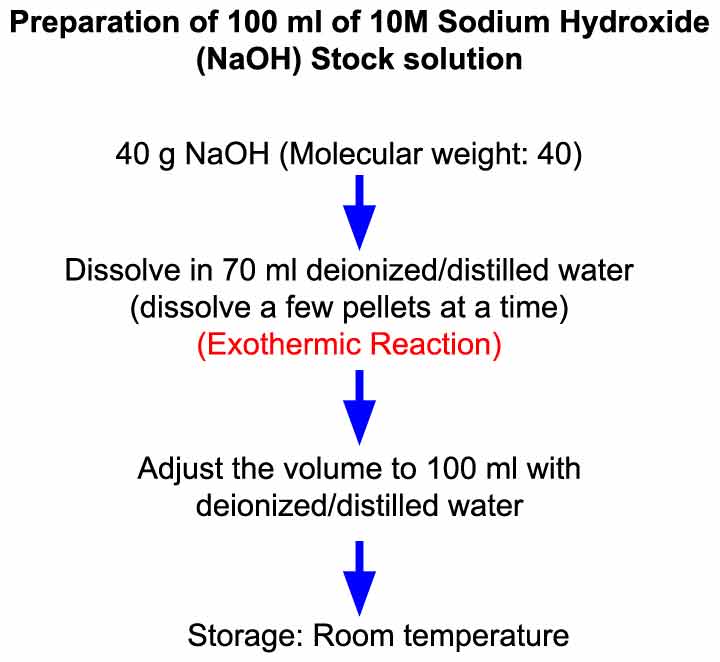
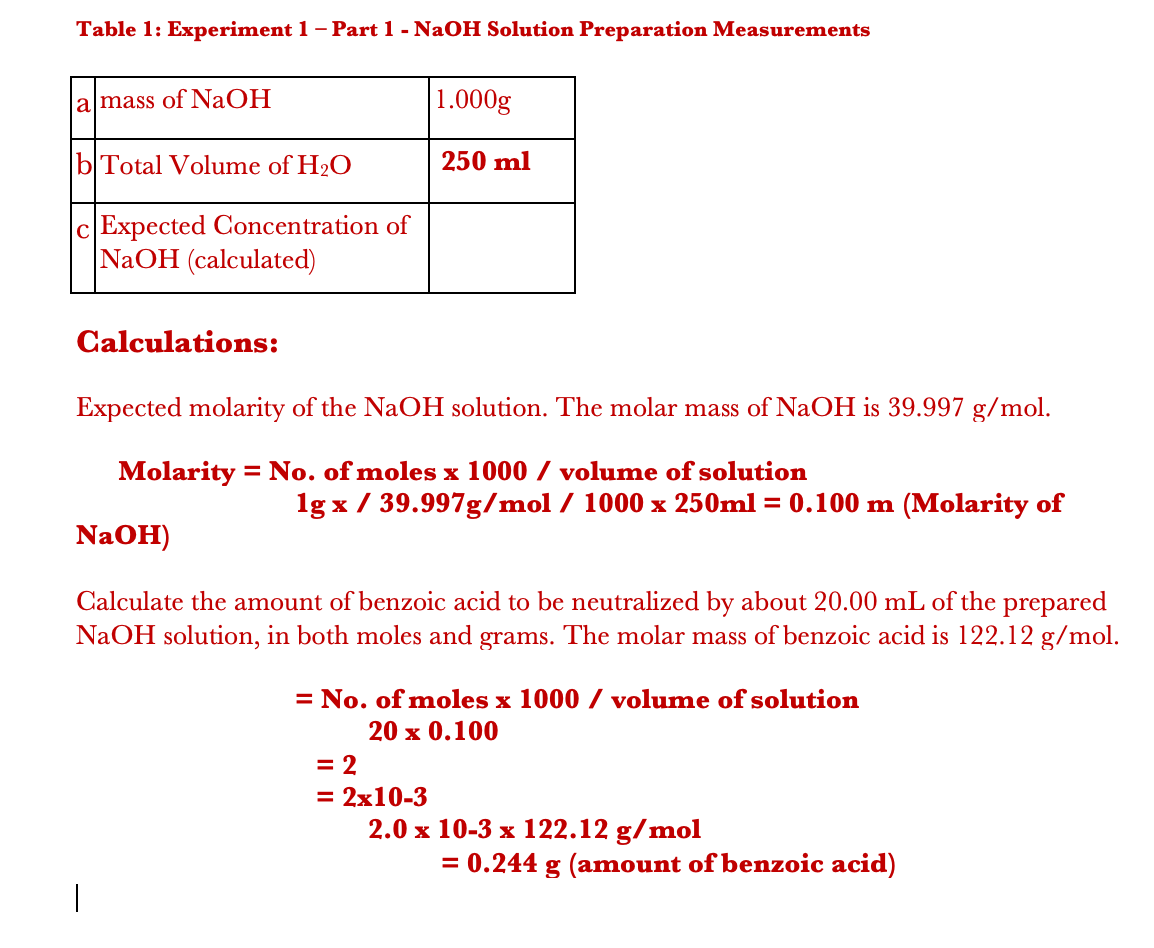
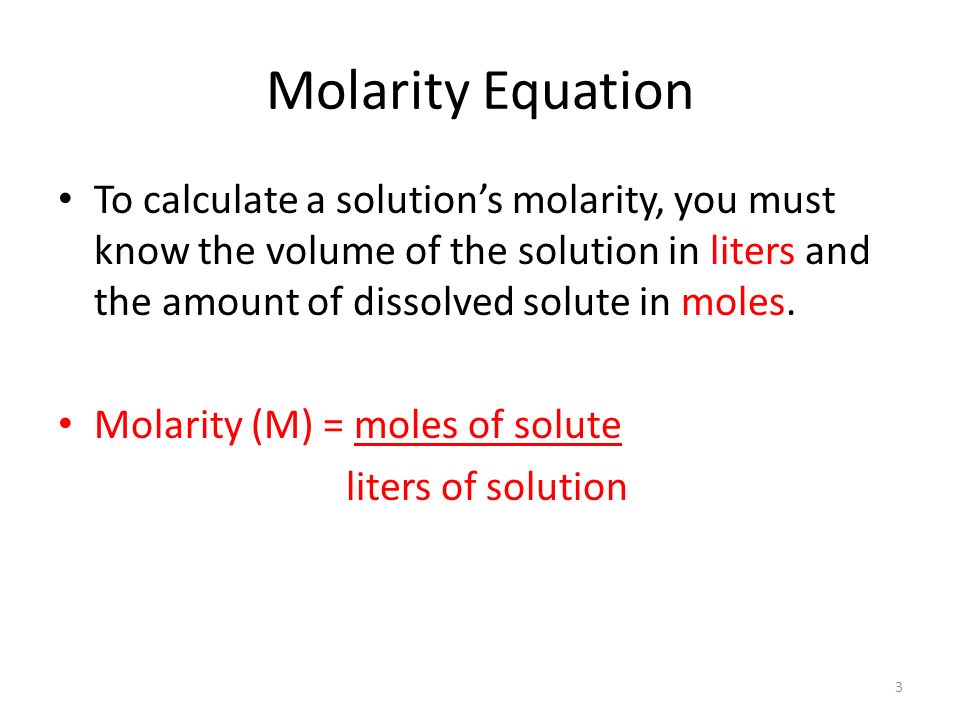
How to prepare 1% sodium hydroxide (NaOH), 5% NaOH, 10% NaOH solutions: Calculation and Explanation - YouTube
![Calculate the mole fraction of NaOH in 10% w/w aqueous solution. [At. MassH=1, O=16, Na=23u] - Brainly.in Calculate the mole fraction of NaOH in 10% w/w aqueous solution. [At. MassH=1, O=16, Na=23u] - Brainly.in](https://hi-static.z-dn.net/files/da5/28dabad35d0225715bc998463bc22ff5.jpg)
Calculate the mole fraction of NaOH in 10% w/w aqueous solution. [At. MassH=1, O=16, Na=23u] - Brainly.in

100mL` of `10% NaOH(w/V)` is added to `100 mL` of `10% HCI(w/V)`. The nature of resultant solut... - YouTube

How to prepare N/10 NaOH solution - Chemistry - Some Basic Concepts of Chemistry - 7762175 | Meritnation.com

SOLVED: Calculate the pH of a 6.7 X 10-2 M NaOH solution. ( a strong base) ( Your answer should have 2 digits after the decimal)

Calculate the molarity of NaOH in the solution prepared by dissolving its 4g in enough water ..... - YouTube



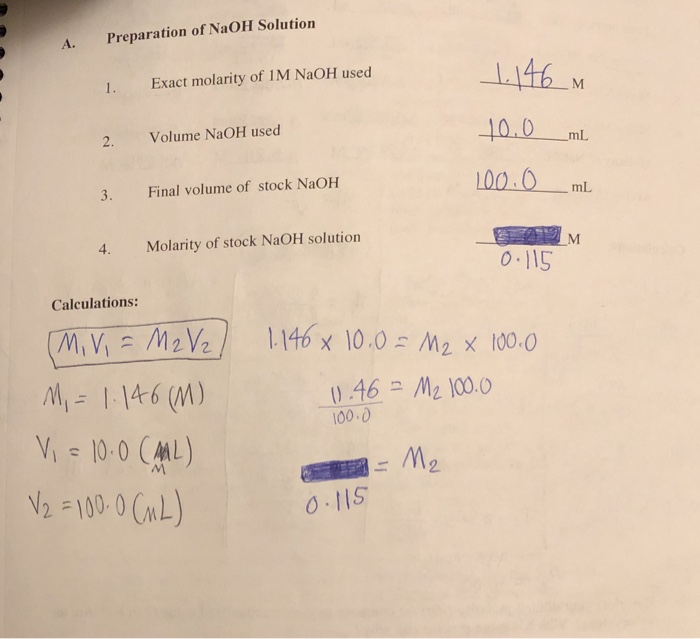


:max_bytes(150000):strip_icc()/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)