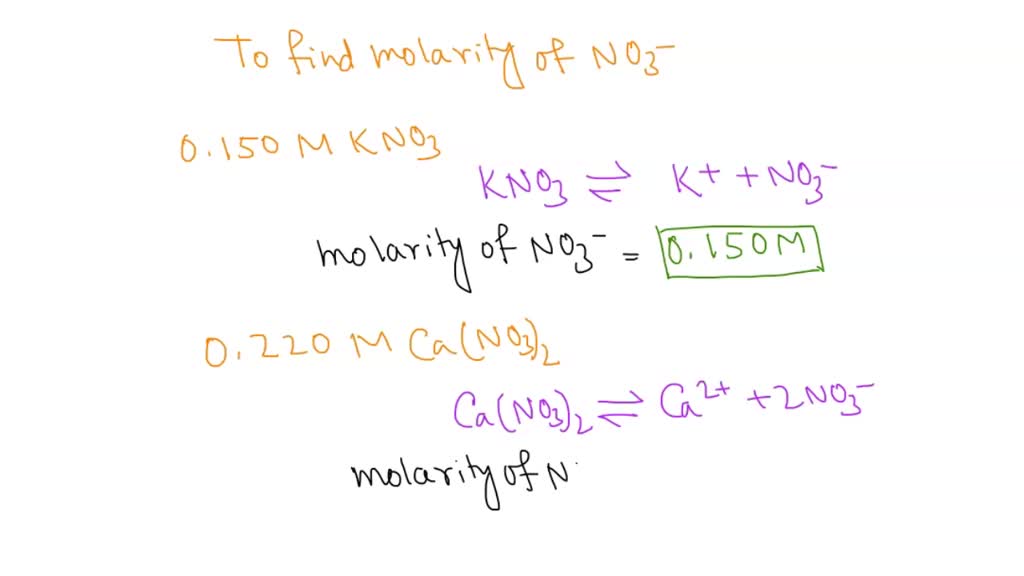
SOLVED: What is the molarity of NO?3 in each solution? 0.150 M KNO3. 0.220 M Ca(NO3)2 0.370 M Al(NO3)3.

Converting molarity to mole fraction, mass percent and molality: A 0.750 M solution of H(2)SO(4) in water has a density of 1.049 g mL^(-1) at 20 ^(@)C. What is the concentration of




:max_bytes(150000):strip_icc()/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png)